Click here for more cases of posterior MI, and more explanation
Case 1.
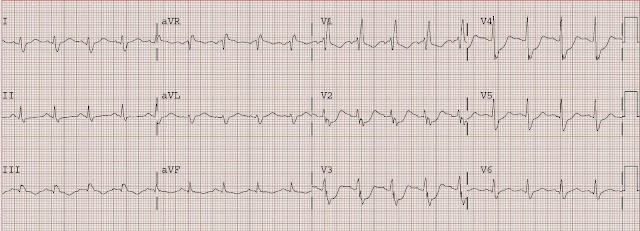
This is a 44 yo male with h/o HTN, CABG at age 34, and 2 drug eluting stents to the first diagonal graft 3 months prior. He presented at 1810 with chest pain 5 days after stopping clopidogrel (on the instructions of another physician). The following ECG (#1) was recorded, with ECG #2 as his baseline.
#1, presenting ECG
#2, baseline from 3 months prior
#1 shows new marked ST depression in V2 and V3.
The differential diagnosis is subendocardial ischemia with probably NSTEMI vs. posterior STEMI.
By ECG alone, posterior MI is more likely because of maximal ST depression in right, as opposed to left, precordial leads. However, given the history of severe 3 vessel coronary disease it is prudent to start with medical management, then observe for resolution of pain, but to also obtain an immediate echocardiogram to ascertain which wall is involved. If pain or ECG findings do not resolve, or if there is posterior wall motion abnormality, then immediate angiography with PCI is indicated.
In this case, maximal medical therapy was undertaken with Aspirin, clopidogrel, heparin, eptifibatide, metoprolol, and IV nitroglycerin and the patient was admitted to the CCU with cardiology consulted immediately. Chest pain resolved and angiogram was not done until the next day. It showed a 100% re-occluded SVG stent to D1; thrombus was suctioned out. Echo confirmed posterior wall motion abnormality. Max troponin was 47.
Case 2.
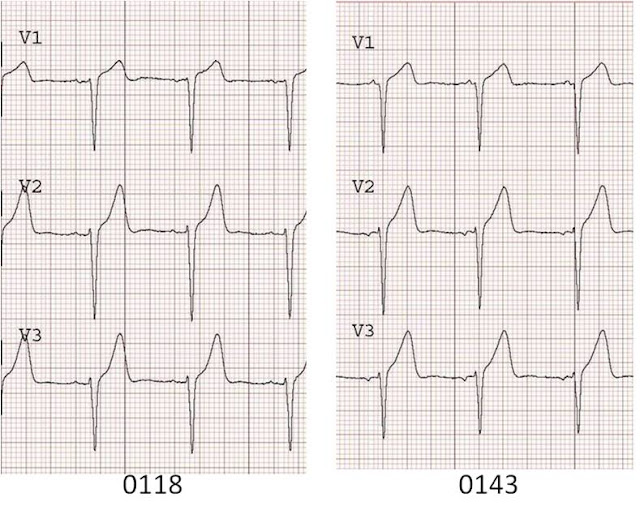
54 yo female with h/o smoking, DM, HTN, c/o chest pain for 3 hours, substernal radiating to both shoulders. She has an initial ECG which shows diffuse ST depression and she was treated for NSTEMI. She developed some pulmonary edema after metoprolol. ECG #2 was recorded:
ST depression is maximal from V2 to V4. This is due to posterior STEMI until proven otherwise. Unfortunately, it was treated as an NSTEMI for 2.5 hours until an echocardiogram showed a posterior wall motion abnormality. Cath revealed a 100% occluded circumflex. This was opened and stented, with the convalescent ECG below, which shows large upright posterior reperfusion T waves with a long QT.
How can such delay be avoided? By recording posterior leads V7-V9, at the level of the tip of the scapula and at the posterior axillary line (V7), a position midway between this and the spine (V8), and a paraspinal lead (V9). Also, obtaining a stat echo. Most of all, recognize this ECG pattern as being by far more likely to be posterior STEMI than due to subendocardial ischemia, which has maximum ST depression in leads V4-V6.
Case 3.
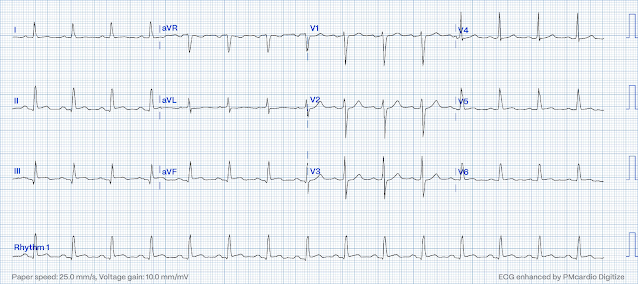
Here is a case of a 66 yo male with a history of CAD who presents with 1.5 hours of chest pain and is stable.
There is ST depression maximal in, again, V2-V4. The vast majority of posterior STEMI is not isolated, but rather concurrent with inferior and/or lateral involvement. In this case there is subtle ST elevtion in the inferior leads, not noticed by the treating physicians. Compare these ST segments with those of the post reperfusion ECG below:
The patient had rapid PCI of a 100% acutely thrombotically occluded mid circumflex which was opened and stented. There was 3 vessel disease.
How common is posterior STEMI and what is the significance of precordial ST depression in ACS?
There are many studies that indirectly reveal that the percent of
STEMIs that are isolated posterior is between 3 and 11% (about 8%). More recently,
a substudy of the recent TRITON-TIMI 38 trial comparing Prasugrel to
Clopidogrel for ACS enrolled 13,608 patients; 1198 had isolated ST
depression in V1-V6. Of these, 314 (26%) had occlusion (TIMI 0 or 1
flow) of the infarct-related artery (i.e., STEMI).
Reference and link to full text below.
There
were 3534 other STEMIs in this study, not including the 314 with ST
depression only (most probably posterior STEMI, but this is not
specified). Add these 314 to the 3534 and you have 314/3848 (8.1%) of
STEMI, many of which have pure isolated posterior STEMI. This conforms with the
previous smaller studies. Moreover, the cath was done a median of 29.4
hours after presentation, so this does not account for those arteries
that spontaneously reperfused (about 25% of STEMI will reperfuse with
antiplatelet and antithrombotic therapy alone within one day -- old
data). Thus, there were probably even more occluded arteries.
Only
14/314 (4.5%) were interpreted by the investigator as STEMI. None of
the patients with an occluded artery had an ECG to PCI time less than 6
hours.
This is not a "rare" event, as I have heard cardiologists claim.
However, note that 3/4 of patients with precordial ST depression did NOT have an occluded artery. Thu, it is very important that you critically evaluate precordial ST depression and do not assume it is posterior STEMI. You will be wrong often, and I have seen many false positive cath lab activations based on a too simplistic approach.
Are there any solid criteria to help provide a DDx between anterior or subendocardial ischemia and posterior STEMI?
This is a very good question, and not easily answered.
First, you should know that when there is precordial ST depression due to subendocardial ischemia, it is not necessarily due to anterior wall ischemia. Data from stress testing shows that subendocardial ischemia DOES NOT LOCALIZE on the ECG, and usually is in leads II, III, aVF and V4-V6. But, again, this does not tell you which artery is involved.
Second, ST depression in V1-V3, vs. V4-V6, is much more likely to be posterior than subendocardial ischemia.
Third, patients at higher risk of NSTEMI (older, more risk factors, h/o angiogram with multivessel disease) are much more likely to have subendocardial disease (vs. younger smoker).
Fourth, patients with reasons to have demand ischemia (tachycardia, sepsis, GI Bleed, etc.) are much more likely to have subendocardial ischemia (like in a stress test); those with posterior MI are much more likely to present with onset of chest pain and with normal vital signs.
Fifth, look for tall R-waves in V1-V3 (the analog of Q-waves in other locations).
Sixth, placement of posterior leads (take leads V4-V6 and place them at the level of the tip of the scapula, with V4 placed at the posterior axillary line ("V7"), V6 at paraspinal area ("V9"), and V5 ("V8") between them.
At least 0.5 mm of ST elevation in just one lead appears to be the most sensitive and specific single "criteria" for posterior MI.
Posterior leads may be falsely negative.
See these cases:
http://hqmeded-ecg.blogspot.com/2013/01/precordial-st-depression-what-is.html
http://hqmeded-ecg.blogspot.com/2011/05/posterior-leads-fail-to-diagnose.html
http://hqmeded-ecg.blogspot.com/2014/12/do-not-confuse-diagnosis-of-posterior.html
Seventh, an immediate echocardiogram can make the distinction
Eighth. Whether or not it is STEMI, the cath
lab should be activated if the ischemia cannot be controlled medically:
aspirin, nitro, beta blockers, clopidogrel, heparin/enoxaparin, GP
IIb/IIIa inhibitor.
The T-wave in posterior STEMI
Notice
also that in the first (pre-reperfusion) ECG of case 3, the T wave is
not upright as in previous cases. Some authors claim that the T wave
must be upright for posterior STEMI. Evidence for this is lacking. In
fact, an upright T wave probably indicates reperfusion of the posterior
STEMI, whereas an inverted T wave is the analog of a hyperacute T wave
(still occluded) but recorded from the opposite side. Either can be
present in posterior STEMI, depending on the state of microvascular
perfusion due to recanalization or to collateral circulation.
Here is a short summary of data on posterior leads, from:
Critical Decisions in Emergency and Acute Care Electrocardiography. William Brady and JD Truwit, editors. Blackwell Publishing 2009. (Smith SW as editor of section on Acute
Coronary Syndromes). This is a quoted excerpt from a chapter that was
written by Daniel T. O’laughlin, MD, and edited by me.
Posterior Lead Orientation and
Diagnostic Criterion
The
posterior precordial leads are positioned in the 5th intercostal
space at the same horizontal line as V6.
Lead V7 is placed at the posterior axillary line, V8 just below the tip
of the scapula and V9 at the paravertebral border.1
STE up to 0.5mm measured at the J point relative to the PR segment in
all three leads can be normal.2
Wung and Drew evaluated the posterior ST segment changes during
PTCA of
the LCX and determined that utilizing a criterion of STE of greater than
or equal to 0.5mm, rather than greater than or equal to 1mm,
demonstrated a sensitivity of 94% for detecting LCX occlusion related
STE.3
This is compared to a sensitivity of 49% when the criterion was greater than or equal to 1mm STE. Conversely, Matetzky et al. showed 100%
specificity for posterior MI of STE greater than or equal to 0.5mm in at least one posterior lead. In Wung’s study, 81% of patients with greater than or equal to 1mm
STE in posterior leads also had other significant STE on the 12-lead ECG, and
96% had some ST deviation.3 However, 22-39% of patients experiencing
posterior MI who have greater than or equal to 0.5mm STE in the posterior leads do not demonstrate STD
in V1-V3.3-5
1. Kligfield
P, Gettes LS, Bailey JJ, et al. Recommendations for the Standardization and
Interpretation of the Electrocardiogram: Part I: The Electrocardiogram and Its
Technology: A Scientific Statement From the American Heart Association
Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology;
the American College of Cardiology Foundation; and the Heart Rhythm Society
Endorsed by the International Society for Computerized Electrocardiology.
Circulation 2007;115(10):1306-24.
2. Taha B, Reddy S, Agarwal J, Khaw K.
Normal limits of ST segment measurements in posterior ECG leads. J
Electrocardiol 1998;31 Suppl:178-9.
3. Wung SF, Drew BJ. New
electrocardiographic criteria for posterior wall acute myocardial ischemia
validated by a percutaneous transluminal coronary angioplasty model of acute
myocardial infarction. Am J Cardiol 2001;87(8):970-4.
4. Matetzky S, Friemark D, Feinberg MS, et
al. Acute myocardial infarction with isolated ST-segment elevation in posterior
chest leads V7-V9: "hidden" ST-segment elevations revealing acute
posterior infarction. J Am Coll Card 1999;34(3):748-53.
5. Matetzky S, Freimark D, Chouraqui P, et
al. Significance of ST segment elevations in posterior chest leads (V7-V9) in
patients with acute inferior myocardial infarction: application for
thrombolytic therapy. J Am Coll Card 1998;31(3):506-11.































.jpeg)
.jpeg)
.jpeg)
.jpeg)
.jpeg)






